Microgravity Could Be the Key to Developing New Therapies for Neurogenerative Diseases
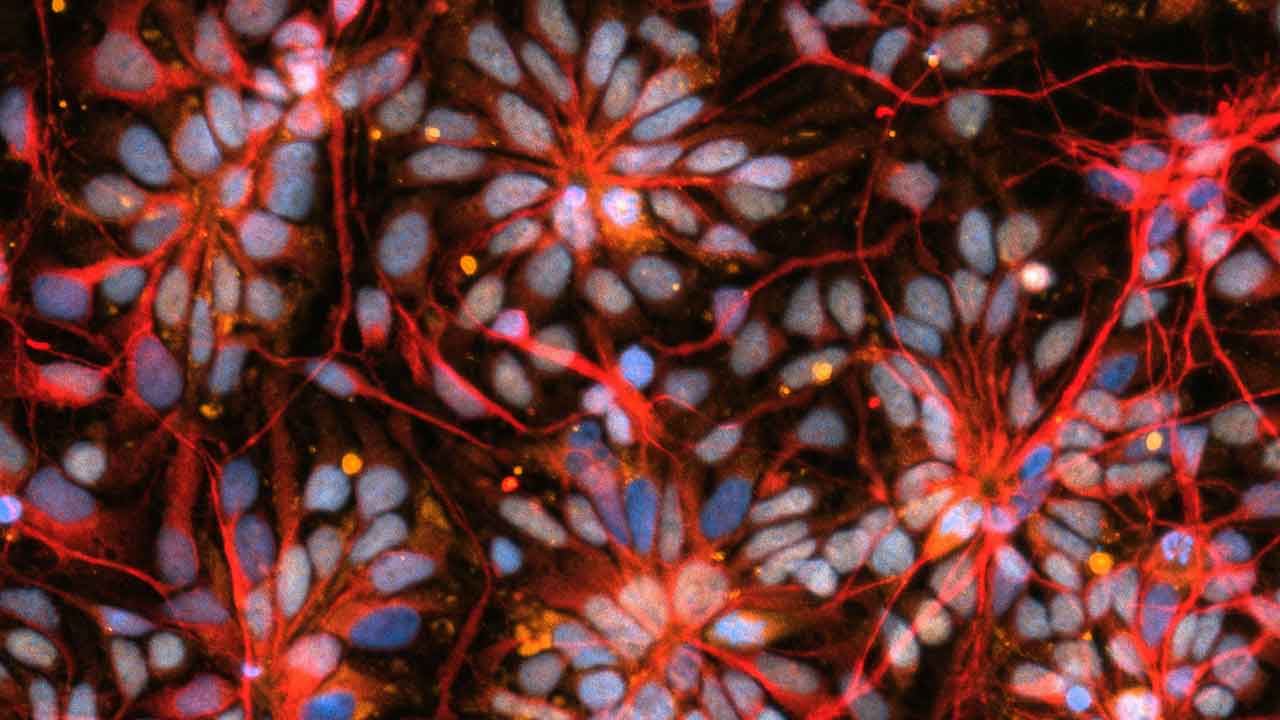
Early stage brain cells from NCSF study of primary progressive MS on SpaceX CRS-30 (March 21, 2024 - April 30, 2024).
Media Credit: Nicolette Pirjanian, NSCF partner lab New York Stem Cell Foundation
NASA Funds Continuation of ISS National Lab-Sponsored Brain Cell Studies From NSCF
April 28, 2025 • By Anne Wainscott-Sargent, Contributing Author
Millions of Americans live with Parkinson’s, multiple sclerosis (MS), and Alzheimer’s—neurodegenerative diseases with no cure and no significant biomarkers to enable early intervention. Fortunately, breakthrough research on the International Space Station (ISSInternational Space Station) offers promising avenues to better understand and fight these devastating diseases.
“Cells mature more rapidly in space, which means you can see what’s happening in an accelerated way. It would take you much longer to see those changes on the ground,” said Paula Grisanti, CEO of the National Stem Cell Foundation (NSCF).
Since 2019, NSCF has conducted six space station investigations using human brain organoids, tiny 3D replicas that mimic how cells behave in the brain. A $3.1 million NASANational Aeronautics and Space Administration award announced this spring will enable NSCF to continue its groundbreaking microgravityThe condition of perceived weightlessness created when an object is in free fall, for example when an object is in orbital motion. Microgravity alters many observable phenomena within the physical and life sciences, allowing scientists to study things in ways not possible on Earth. The International Space Station provides access to a persistent microgravity environment. studies, funding three additional ISS projects through 2027.
In earlier investigations sponsored by the ISS National Laboratory®, the team examined brain organoids made from the cells of people with Parkinson’s disease and primary progressive MS. Together, these two conditions affect at least 2 million people in the U.S.
“These were the first 3D human patient-specific organoid models made from the cells of people with these diseases sent to the International Space Station,” Grisanti said. “In space, you can see cells talking to each other in a way that’s not possible on Earth. It’s providing valuable new insight into how these disorders develop, accelerating biomarker discovery for early diagnosis and opening a whole new door to potential cell, gene, and drug therapies that don’t currently exist for these diseases.”
The upcoming flights will build on the team’s past research by adding organoids made from the cells of people with Alzheimer’s disease. Approximately 6.7 million Americans live with Alzheimer’s, resulting in an annual economic impact of more than $321 billion.
Brain Organoids in Space
NSCF scientists produce brain organoids from induced pluripotent stem cells (iPSCs) derived from human skin cells. “Stem cells mimic the very early stages of development in a mother’s womb and have the potential to become any cell type in the human body,” explained Pinar Mesci, NSCF space project advisor who serves as senior program manager for in-space biomanufacturing at Axiom Space.
Skin cells are turned into stem cells through a process called cellular reprogramming, and the stem cells can then be developed into brain cells.
In previous flights to the International Space Station, the NSCF team found that brain cells mature faster in space. In the upcoming experiments, NSCF scientists will test existing drugs and new ones in development to see if they interfere with disease progression. “The ability to see what happens to these cells in space as they mature is particularly valuable for diseases that are diagnosed later in life,” explained Grisanti.
Studies from stem cell-derived organoid models have significant advantages over traditional animal studies, Mesci said. For her doctorate in neuroscience from Pierre & Marie Curie University in Paris, Mesci used mouse models to study Lou Gehrig’s disease, or ALS, a fatal motor neuron disease.
“Mouse models are good to study certain aspects of diseases, but unfortunately, none of the treatments that we tried in those animals were translatable enough to develop a cure or to significantly treat symptoms in patients,” she explained. Organoids more closely represent how brain cells function in the human body, providing a more robust and accurate model.
Tracing Inflammation Pathways
In the upcoming series of NSCF projects, the team will trace the inflammation pathways involved in neurodegenerative diseases. Findings could help researchers better understand disease onset and identify possible biomarkers.
“Unfortunately, studies have been done for many decades but have yet to lead to any treatment options, mainly because we lack a good biomarker,” Mesci said. “Biomarkers allow us to identify damage before the disease gets to the point that symptoms appear, at which stage it’s often already too late to intervene. For instance, in a disease like ALS, which is very aggressive, patients die within two to five years after symptoms begin.”
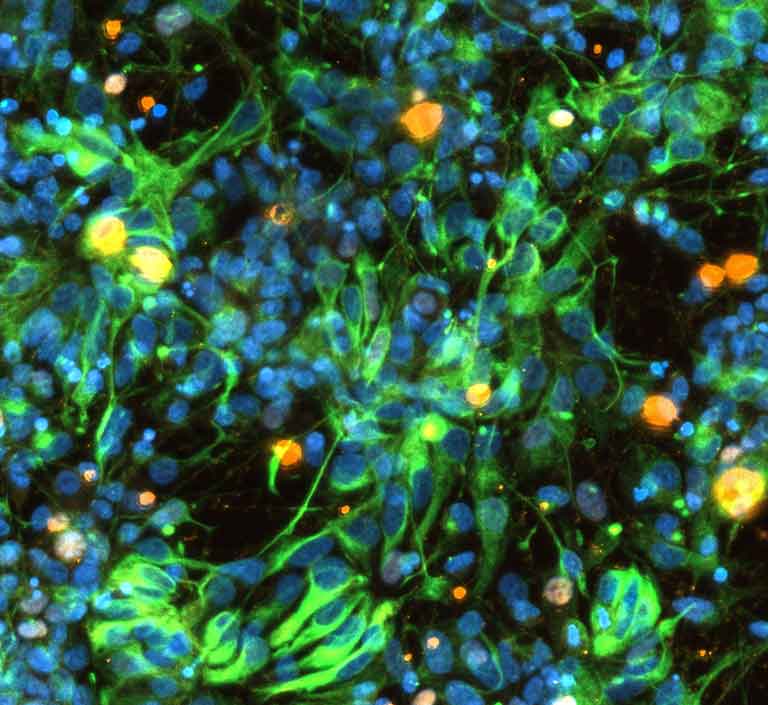
Early stage brain cells from NSCF study of Parkinson's disease on SpaceX CRS-30 (March 21, 2024 - April 30, 2024).
Media Credit: Nicolette Pirjanian, NSCF partner lab New York Stem Cell Foundation
In the case of Parkinson’s disease, once tremors begin, significant nerve damage has already occurred. Estimates suggest that by the time symptoms are noticeable, a person may have lost almost 70% of the dopamine-producing cells in their brain, which regulate body functions like movement.
“Once you have a lot of things dying in the brain, chronic inflammation will amplify everything,” said Mesci. Using the analogy of a forest fire, she noted, “It’s much easier to put a fire out when it first starts than when hundreds of acres have already burned.”
Building on Learnings from Past Missions
NSCF will leverage processes developed from earlier space station missions, including techniques for optimizing experiments to focus on the disease pathways. The team hopes to answer questions such as: What are the drivers of these diseases? What can human organoid models tell us about the diseases’ early stages when no symptoms exist? And how can we intervene?
Ultimately, NSCF plans to make its disease models available to pharmaceutical and life science companies for drug discovery and the development of new therapeutic options for neurodegenerative diseases.
“The ability to see and analyze cell interactions in a way not possible on Earth is creating an unprecedented opportunity to accelerate the discovery of new therapies for these and other neurodegenerative diseases that affect tens of millions worldwide,” Grisanti said. “This is a transformative moment in history for finding treatments and cures for some of the most costly and terrible diseases of our time.”
